Abstract
Background: Early diagnosis of AL amyloidosis remains a diagnostic challenge, mainly due to the low prevalence of the disease and heterogeneity of presentation. Artificial intelligence (AI) is a tool which can assist in improving disease recognition.
Objective: To establish an AI-based model to accurately discriminate patients with AL amyloidosis from patients with monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM), who are at risk of developing AL amyloidosis.
Material & Methods: We screened all consecutive patients from the dysproteinemia database at Mayo Clinic Rochester with the diagnoses of MGUS, SMM and AL amyloidosis who were diagnosed between January 1, 2000, and December 31, 2018 and evaluated within 90 days of their diagnosis. Patients who had more than one diagnosis were excluded. The choice of variables to be included in the models were those in whom data was available in >50% of the patients (with one exception being serum free light chain assay which was available in 44% of patients but deemed important for modeling). The variables included in the modeling were the following: Age, gender, height, weight, systolic blood pressure, diastolic blood pressure, heart rate, hemoglobin, MCV, white blood count, absolute lymphocyte count, platelet count, creatinine, albumin, calcium, SGOT, alkaline phosphatase, total bilirubin, serum free kappa and serum free lambda. In a subset model, we incorporated the AI-enhanced electrocardiogram (AI-ECG) score for cardiac amyloidosis (available in routine clinical practice at Mayo Clinic). This variable is AI-based captures from standard 12-lead ECG to predict the probability of cardiac amyloidosis.1 We randomly divided the final study cohort in a 1:1 ratio to training and validation set. Random forest algorithm was used for modeling to predict the probability of AL amyloidosis diagnosis versus non-AL amyloidosis diagnosis (i.e., MGUS/SMM).
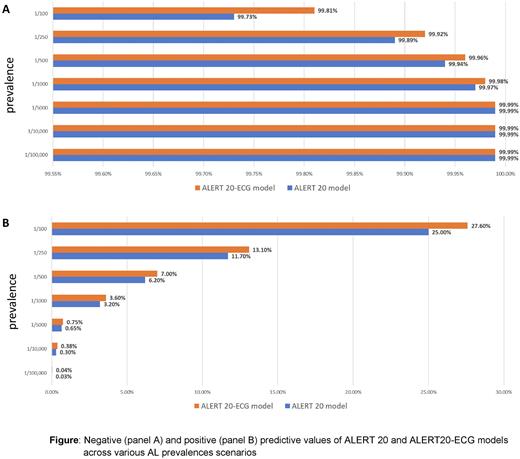
Results: 20, 579 patients were screened, of which 4,087 patients (19.9%) were included in this study (MGUS n=2716, AL amyloidosis n=1217, SMM n=154). The first model (ALERT 20) included the above-mentioned 20 variables, of which 7 were clinical and 13 were common blood count and blood chemistry testing. The validation set yielded an AUC-ROC of 0.946 for accurate classification of AL amyloidosis against MGUS/SMM. The second model (ALERT 20-ECG model) included all variables used in the ALERT 20 mode plus the AI-ECG score. In this model, 3090 patients were included, after excluding patients with missing ECG (MGUS n=1896, AL amyloidosis n=1075, SMM n=119). By adding the AI-ECG score, the AUC-ROC in the validation set for accurate disease classification increased modestly to 0.958. Analysis of the relative contribution of each variable to model performance identified serum free light chains, serum albumin and AI-ECG as the most influential variables. As disease prevalence decreases the positive predictive value (PPV) and increases the negative predictive value (NPV) of a model, we tested the models’ performance in various prevalence scenarios, including the estimated prevalence of AL amyloidosis among MGUS/SMM patients (estimated at 1/100-1/1000 person years). Although the PPVs of ALERT 20 and ALERT 20-ECG were low (<1-27.6%), the NPV was over 99% (Figure).
Summary & Conclusion: Two AI-based algorithms to aid clinicians in recognizing patients with AL amyloidosis and distinguishing them from patients with MGUS/SMM are presented. These models, when tested in populations at risk of AL amyloidosis (where the prevalence of AL amyloidosis is sufficiently elevated), have a plausible PPV to be used as screening tool for this uncommon, yet life-threatening, disease.
Disclosures
Muchtar:Janssen: Honoraria; Protego: Consultancy. Gertz:Ionis/Akcea: Other: personal fees; Prothena: Other: personal fees; Sanofi: Other: personal fees; Janssen: Other: personal fees; Aptitude Healthgrants: Other: personal fees; Ashfield: Other: personal fees; Juno: Other: personal fees; Physicians Education Resource: Other: personal fees; Abbvie: Membership on an entity's Board of Directors or advisory committees, Other: personal fees from Data Safety Monitoring Board; Johnson & Johnson: Other: personal fees; Celgene: Other: personal fees; Research to Practice: Other: personal fees; Sorrento: Other: personal fees; i3Health: Other: Development of educational materials for i3Health. Lacy:Celgene: Research Funding. Dingli:Sanofi S.A.: Consultancy; Takeda Pharmaceuticals: Consultancy; Novartis: Consultancy; Janssen Pharmaceuticals: Consultancy; GlaxoSmithKline: Consultancy; Bristol Myers Squibb: Consultancy; Apellis Pharmaceuticals: Consultancy; Alexion Pharmaceuticals: Consultancy. Kourelis:Novartis: Research Funding. Friedman:Marani Health: Current equity holder in private company, Other: IP licensed for fetal monitoring; Anumana: Membership on an entity's Board of Directors or advisory committees, Other: AI ECGs algorithms licensed; Boston Scientific: Other: Steering committee, advisory group: funds to Mayo Clinic; Alive Cor: Other: AI algorithms licensed; MediCool: Current equity holder in private company, Current holder of stock options in a privately-held company, Other: Leadership; xAI.health: Current equity holder in private company, Other: Leadership; Medtronic: Other: EV ICD steering committee, funds to Mayo Clinic; Eko Health: Other: AI algorithms licensed. Kumar:AbbVie,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive,: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE,: Research Funding; MedImmune/Astra Zeneca,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck,: Research Funding; Novartis,: Research Funding; Roche: Research Funding; Sanofi: Research Funding; Oncopeptides: Other: Independent review committee. Dispenzieri:Janssen: Membership on an entity's Board of Directors or advisory committees; Oncopeptides, and Sorrento: Other: Data monitoring safety committee; Alynlam, Pfizer, Takeda, and BMS: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal